Density Altitude Calculator
Updated in November 2023 to include the effect of humidity in the density altitude calculation.
Use the toggle switch to deselect the dew point temperature if unknown. The calculation will then proceed with a dry air approximation.
The Importance of Density Altitude in Aviation
Our atmosphere is a dynamic system with variations in pressure, temperature, humidity and precipitation typically seen throughout the day across a relatively small area. These changes in the local environmental conditions cause associated changes to the air density, which plays a crucial role in determining aircraft performance.
Air Density and Aircraft Performance
An increase in the air density is associated with an improvement in both the aerodynamic performance and the thrust generated by an aircraft. Conversly, a reduction in the air density results in less engine power being produced, and less lift generated by the wings, which will result in a loss in performance at critical phases in flight such as takeoff.
- A drop in air density results in less lift being produced by a wing at a given airspeed and angle of attack.
- A drop in air density results in a loss in power generated by the engine (especially true in normally aspirated general aviation aircraft engines).
- Propeller driven aircraft will see an additional loss in thrust due to a drop in air density as a result of the propeller being unable to extract as much thrust at a given RPM (a propeller acts in the same way as a wing with an airfoil section designed to generate a force due to the pressure variation over the upper and lower surfaces).
Losses in aircraft performance are most noticable during takeoff and climb out at high altitudes, and on hot days.
Factors Affecting Air Density
Air density is driven by four primary factors. These are altitude, local variations in air pressure, local temperature variations and humidity.
- Altitude: An increase in altitude results in a decrease in air density with a corresponding drop in static pressure and temperature.
- Local pressure variations: regions of high and low pressure associated with local weather phenomena result in density variations. Higher pressures result in more dense air and lower pressures less dense air.
- Local temperature variations: higher temperatures are associated with lower air density, and cooler temperatures with higher air density.
- Humidity: Moist air is less dense than dry air, and so increased humidity results in a decrease in air density.
The Standard Atmosphere Model
The atmosphere is in a constant state of flux due to global and local weather phenomena. Since local conditions vary at all places and at all times aeronautical engineers work with a theoretical atmosphere model when performing aircraft performance analysis calculations such as those that appear in aircraft Pilot Operating Handbooks. This atmosphere model is referred to as the International Standard Atmosphere and provides a mathematical model describing how pressure, temperature, density and viscosity vary over a range of altitudes from sea level to 80 km.
The AeroToolbox Standard Atmosphere Calculator has proven to be a popular tool which outputs the relevant atmospheric properties given an altitude and temperature, or temperature offset from standard.
Sea level standard conditions are very important from an aviation stand point. For example, airspeed indicators are calibrated on the assumption that the aircraft is operating at sea level on a standard day.
| Temperature | 15 °C |
| Pressure | 1013.25 hPa |
| Density | 1.225 kg/m³ |
Of course there are very few instances when an aircraft is actually operating in these standard conditions, and as such the true airspeed will usually differ from the indicated airspeed — oftentimes by a substantial amount. You can play around with our Airspeed Conversion Tool to see this in action. The tool is especially useful when wanting to calculate true airspeed given indicated airspeed and an operating altitude.
So What then is Density Altitude?
Density altitude is simply the altitude in the Standard Atmosphere model that corresponds to the actual air density currently being experienced. This is a convenient and intuitive way of describing the air density as it gives the pilot an "equivalent altitude" at which he or she is operating.
For example, Johannesburg South Africa sits at an altitude of approximately 5000 ft above sea level. However, in the summer the temperatures routinely reach 30+ Celcuis with afternoon thunderstorms. A hot and humid summer day may result in a density altitude in excess of 9000 ft which severly degrades aircraft performance.
Performance Charts
To show the importance of the prevailing atmospheric conditions on aircraft performance you only need to open any aircraft POH to the section dedicated to takeoff performance. The example below is from a Piper PA-28-121 Archer II manual and plots the variation in takeoff performance with pressure altitude and operating air temperature at a flaps 25 setting. The pressure altitude corrected for the operating air temperature is of course the density altitude.
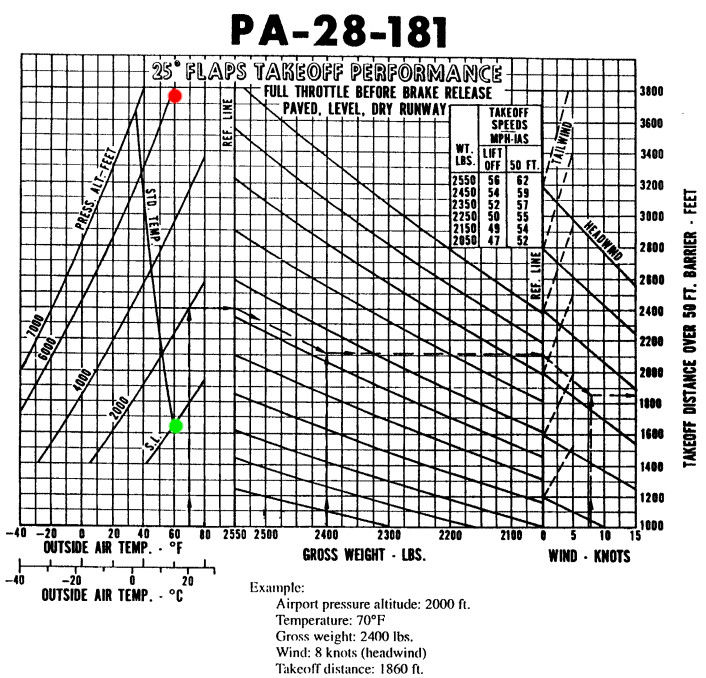
- On a hot day with pressure altitude of 6000 ft, the gross weight takeoff run in windless conditions is approximately 3700 ft.
- A standard day at sea level (15°C) at gross weight in windless conditions results in a takeoff run closer to 1600 ft. This is less that half of the hot and high takeoff run!
This is a great example of the effect that density altitude has on performance. An airport at 5000 ft elevation with an outside air temperature of 32°C has a density altitude in excess of 8000 ft. This is why it is so important to know and understand your aircraft and it's performance limitations before embarking on a flight — especially at or near gross weight on a hot day at higher altitudes.
Calculation Methodology
Pressure Altitude
The pressure altitude \( \left( \textnormal{PA} \right) \) is determined from the airfield elevation and the local QNH using a formula published by the National Oceanic and Atmospheric Administration (NOAA).
The altitude output is in feet and the QNH value must be converted to milibar (mb) before being used in the equation.
$$ \textnormal{PA} = \textnormal{Field Elevation} + 145366.45 \left[ 1 - \left(\frac{\textnormal{QNH}_{mb}}{1013.25}\right)^{0.190284} \right]$$
To convert QHN from inches of mercury to milibar:
$$ \textnormal{QNH}_{mb} = \textnormal{QNH}_{inHg} \times 33.86388 $$
QNH is often given in hectopascal (hPa) which is the same an millibar.
$$ \textnormal{QNH}_{mb} = \textnormal{QNH}_{hPa} $$
This is the altitude in the standard atmosphere model that has the same atmospheric pressure as the current atmospheric pressure in the region of interest (usually an airfield).
Density Altitude
To calculate the density altitude you first need to determine current air density based on the prevailing atmospheric conditions.
Density is simply the mass per unit volume, and in our case we must account for any water vapour present in the local atmosphere, which has the impact of reducing the overall air density.
Why does the presence of water vapour in the atmosphere reduce density of air?
The answer lies in the composition of air, which is comprised predominently of nitrogen \( \left( N_{2} \right) \) and oxygen \( \left( O_{2} \right) \). The atomic weight of nitrogen is 14, which means that the \( N_{2} \) molecule has a weight of 28. Similarly, the weight of an oxygen molecule is 32. A single water molecule \( \left( H_{2}0 \right) \) has an atomic weight of 18, so that every water molecule that displaces a nitrogen or oxygen molecule in the air results in a corresponding drop in the density.
Density Equation
The density of humid air (water vapour present) can be estimated as:
$$ \rho_{Air} = \left(\frac{P_{d}}{R_{D} T}\right) + \left( \frac{P_{v}}{R_{V}T}\right) $$
| Symbol | Value | Units | Description |
|---|---|---|---|
| \( \rho_{Air} \) | - | \( kg/m^{3} \) | Air density |
| \( P_{d} \) | - | \( Pa \) | Partial pressure of dry air |
| \( P_{d} \) | - | \( Pa \) | Partial pressure of water vapour |
| \( T \) | - | \( K \) | Air temperature in Kelvin |
| \( R_{D} \) | 287.05 | \(J/(kgK) \) | Gas constant dry air |
| \( R_{V} \) | 461.495 | \( J/(kgK) \) | Gas constant water vapour |
In order to apply the equation above we need to determine the partial pressure of dry air and the pressure of the water vapour present in the air.
Vapour Pressure
The vapour pressure is determined from the dew point temperature, using a polynomial curve fit that models the saturation vapour pressure. This is known as the Herman Wobus approximation.
$$ E_{s} = e_{so} / p^{8} $$
where:
$$ p = \left( c_{0} + T \left(c_{1}+T \left( c_{2}+T \left( c_{3}+T \left( c_{4}+T \left( c_{5}+T \left( c_{6}+T \left( c_{7}+T \left( c_{8}+T \left( c_{9} \right)\right)\right)\right)\right)\right)\right)\right)\right)\right) $$
| Symbol | Value | Units | Description |
|---|---|---|---|
| \( E_{s} \) | - | \( mb \) | Saturation pressure of water vapour |
| \( e_{so} \) | 6.1078 | polynomial constant | |
| \( T \) | - | \( C \) | Temperature Celcius |
The values of the ten polynomial coefficients are shown below.
| \( c_{0} \) | 0.99999683 | \( c_{1} \) | 0.90826951E-02 |
| \( c_{2} \) | 0.78736169E-04 | \( c_{3} \) | 0.61117958E-06 |
| \( c_{4} \) | 0.43884187E-08 | \( c_{5} \) | 0.29883885E-10 |
| \( c_{6} \) | 0.21874425E-12 | \( c_{7} \) | 0.17892321E-14 |
| \( c_{8} \) | 0.11112018E-16 | \( c_{9} \) | 0.30994571E-19 |
Substitute the dew point temperature in Celcius into the Wobus polynomial to determine the partial pressure of water vapour in the air.
$$ P_{v} = e_{so} / p^{8} $$
Dry Air Partial Pressure
Next you need to calculate the partial pressure of dry air \( P_{d} \).
The easiest way to do this is to determine the absolute pressure \( P_{a} \) (also known as station pressure) from the local QNH, from which the partial pressure of dry air follows.
$$ P_{a} = P_{v} + P_{d}$$
| Symbol | Description |
|---|---|
| \( P_{a} \) | Absolute or Station Pressure |
| \( P_{v} \) | Partial Pressure of Water Vapour |
| \( P_{d} \) | Partial Pressure of Dry Air |
Absolute or Station Pressure
The relationship between actual pressure \( P_{a} \) and QNH can be determined from the equation below:
$$ P_{a} = \left( QNH^{k1} - \left( k2 \times h \right) \right)^{\frac{1}{k1}}$$
| Symbol | Value | Units | Description |
|---|---|---|---|
| \( P_{a} \) | - | \( mb \) | Absolute pressure |
| \( QNH \) | - | \( mb \) | barometric altitude setting |
| \( k1 \) | 0.190263 | - | Constant |
| \( k2 \) | 8.417286e-5 | - | Constant |
| \( h \) | - | \( m \) | Geopotential station elevation |
The equation above uses the geopotential station elevation which is slightly different to the geometric elevation that would be reported as the airport elevation. Geopotential altitude is a mathematical description based on the potential energy of an object subjected to earth's gravity.
Geometric and Geopotential Altitude
You can convert between geopotential and geometric elevation using the following formula:
$$ Z = \frac{E \times H}{E - H} $$
| Symbol | Value | Units | Description |
|---|---|---|---|
| \( Z \) | - | \( km \) | Geometric Altitude |
| \( H \) | - | \( km \) | Geopotential Altitude |
| \( E \) | 6356.766 | \( km \) | ISA radius of the Earth |
Once you have solved for \( P_{a} \), the partial pressure of dry air follows:
$$ P_{d} = P_{a} - P_{v} $$
You now have everything you need to calculate the density of the humid air (equation repeated below).
$$ \rho_{Air} = \left(\frac{P_{d}}{R_{D} T}\right) + \left( \frac{P_{v}}{R_{V}T}\right) $$
Density Altitude Equation
The density altitude follows directly from the standard atmosphere formulae once the actual air density is known.
$$ \textnormal{h} = \frac{T_{0}}{\Gamma} \left[1 - \left( \frac{R \cdot T_{0} \cdot \rho_{Air}}{M \cdot P_{0}} \right)^{\frac{\Gamma R}{gM-\Gamma R}} \right] $$
| Symbol | Value | Units | Description |
|---|---|---|---|
| \( h \) | - | \( m \) | Geopotential altitude |
| \( \rho_{}Air \) | - | \( kg/m^{3} \) | Calculated Density of Air |
| \( g \) | 9.80665 | \(m/s^{2} \) | Acceleration due to gravity |
| \( M \) | 0.028964 | \(kg/mol \) | Molar mass of dry air |
| \( R \) | 8.31432 | \(J/mol K \) | Universal Gas Constant |
| \( \Gamma \) | 0.0065 | \(K/m \) | Environmental Lapse Rate (valid below 11 000 m) |
| \( T_{0} \) | 288.15 | \(K \) | Sea level Standard Temperature |
| \( P_{0} \) | 101325 | \(Pa \) | Sea level Standard Pressure |
The equation above solves for the geopotential altitude in meters which should be again converted to geometric altitude, corresponding to density altitude. Ensure that you convert the result \( h \) above to \( H \) in km before converting to geometric altitude.
$$ DA = \frac{E \times H}{E - H} $$
Density Altitude Approximation Formulae
A common approximation method for calculating the density altitude is presented below. These equations are generally used by pilots before getting airborne on hot days at or near gross weight. We would suggest rather bookmarking this page and making use of our calculator, but the density altitude calculation as presented here is often used as an exam question during the theory portion of the PPL.
Pressure Altitude Approximation
To convert from airfield elevation to a pressure altitude when the current QNH is known:
$$ \textnormal{PA}_{hPa} \approx \left( 1013.25 - QNH_{hPa} \right) \times 30 ft + \textnormal{field elevation} $$
If the QNH is given in inches of mercury:
$$ \textnormal{PA}_{inHg} \approx \left( 29.92 - QNH_{inHg} \right) \times 1000 ft + \textnormal{field elevation} $$
If you are sitting in your aircraft then the pressure altitude can be quickly determined by setting your altimeter to 1013.25 mb or 29.92 inHg and reading off the resulting altitude.
Density Altitude Approximation
The density altitude can be estimated using the pressure altitude as an input and the simple formula below.
$$ \textnormal{DA} \approx \textnormal{PA} + 120 \left ( T - T_{ISA} \right) $$
Where:
\( T \) is the current Outside Air Temperature in °C.
\( T_{ISA} \) is the standard temperature at the given airport elevation in °C.
The variation in standard temperature with altitude can be approximated between sea level and 36 000 ft by the following equation:
$$ T_{ISA} \approx 15 - \left( 0.00198 \times \textnormal{PA} \right) $$
Approximation Worked Example
Calculate the pressure and density altitude given the following conditions:
\( \textnormal{Airfield Elevation} = 5000 \textnormal{ ft}, \)
\( \textnormal{QNH} = 1018 \textnormal{ hPa}, \)
\( \textnormal{OAT} = 30 ^{\circ}C \)
\( \textnormal{Dry Atmosphere} \)
First approximate the pressure altitude:
$$ \textnormal{PA}_{hPa} \approx \left( 1013.25 - 1018 \right) \times 30 + 5000 = 4858 \textnormal{ ft} $$
Then calculate the temperature deviation from standard:
$$ T_{ISA} \approx 15 - \left(0.00198 \times 4858 \right) = 5.38 ^{\circ}C $$
The approximate density altitude follows:
$$ \textnormal{DA} \approx 4858 + 120 \left (15T - 5.38 \right) = 7812 \textnormal{ ft} $$
Comparison with Calculator
These results compare well to the more accurate analysis performed in the calculator at the top of the page.
| AeroToolbox Calculator | Approximation Formulae | % Difference | |
|---|---|---|---|
| Pressure Altitude (ft) | 4871 | 4858 | 0.3 |
| Density Altitude (ft) | 7644 | 7812 | -2.1 |






